Sodium carbonate reacts with citric acid
Bildnummer 12629581
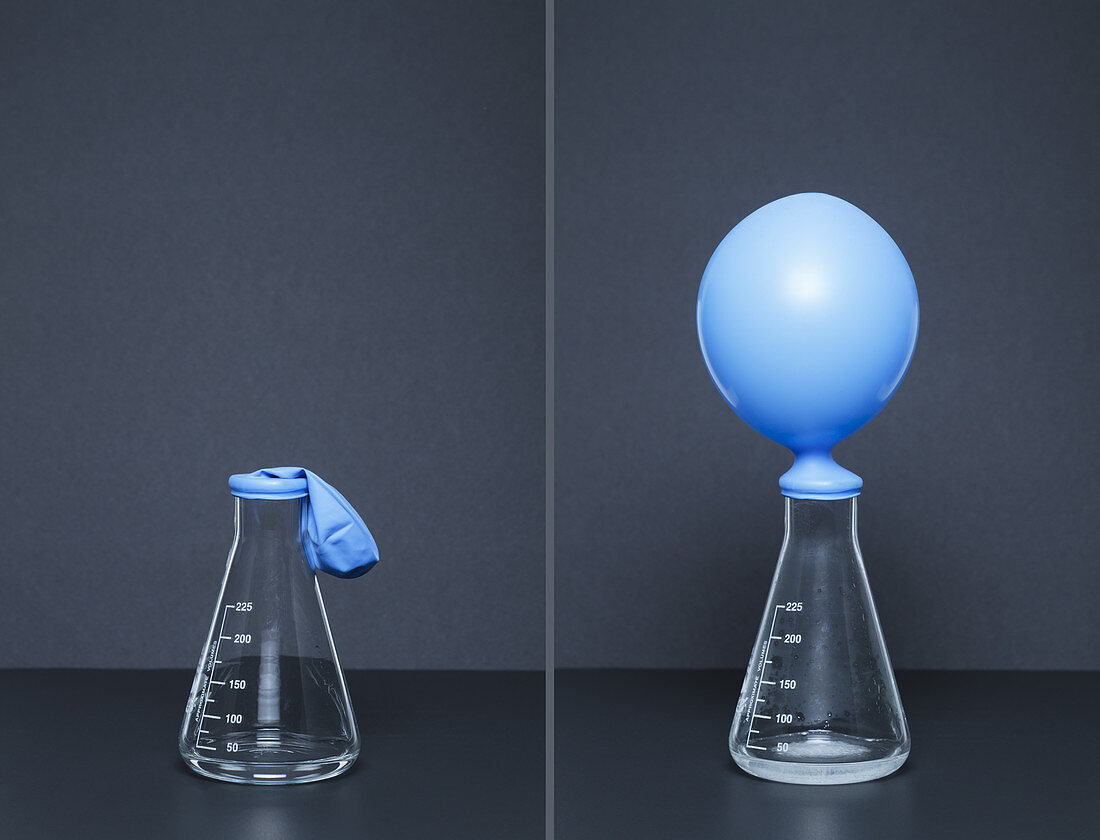
| Sodium carbonate reacts with citric acid. 0.025 mol of sodium carbonate (Na2CO3) is placed in a balloon, which is then attached to an Erlenmeyer flask that contains 20 mL of saturated citric acid (H3C6H5O7), left frame. After the balloon is turned upright, sodium carbonate pours into the acid. In the reaction, H3C6H5O7 + Na2CO3 -> Na3C6H5O7 + CO2 + H2O, carbon dioxide gas is produced and the balloon is inflated as a result, right frame. This is an example of a carbonate-acid reaction, which in itself is a double-replacement reaction followed by a decomposition reaction. | |
| Lizenzart: | Lizenzpflichtig |
| Credit: | Science Photo Library / Giphotostock |
| Bildgröße: | 5700 px × 4354 px |
| Modell-Rechte: | nicht erforderlich |
| Eigentums-Rechte: | nicht erforderlich |
| Restrictions: | - |
Preise für dieses Bild ab 15 €
Universitäten & Organisationen
(Informationsmaterial Digital, Informationsmaterial Print, Lehrmaterial Digital etc.)
ab 15 €
Redaktionell
(Bücher, Bücher: Sach- und Fachliteratur, Digitale Medien (redaktionell) etc.)
ab 30 €
Werbung
(Anzeigen, Aussenwerbung, Digitale Medien, Fernsehwerbung, Karten, Werbemittel, Zeitschriften etc.)
ab 55 €
Handelsprodukte
(bedruckte Textilie, Kalender, Postkarte, Grußkarte, Verpackung etc.)
ab 75 €
Pauschalpreise
Rechtepakete für die unbeschränkte Bildnutzung in Print oder Online
ab 495 €
